A new family of carboxylesterase genes from powdery mildew fungi
Background
Carboxylesterases are a superfamily of enzymes that hydrolyze carboxylesters in natural and synthetic molecules. They can detoxify cells from harmful compounds and metabolites. Genes encoding carboxylesterases are widely present in eukaryotes, including fungi. Catalytic activity of carboxylesterases requires a catalytic triad formed by the residues Ser-His-Glu. Mutations that lead to substitution of the residues of the catalytic triad often result in loss of the catalytic function of carboxylesterases
Major findings
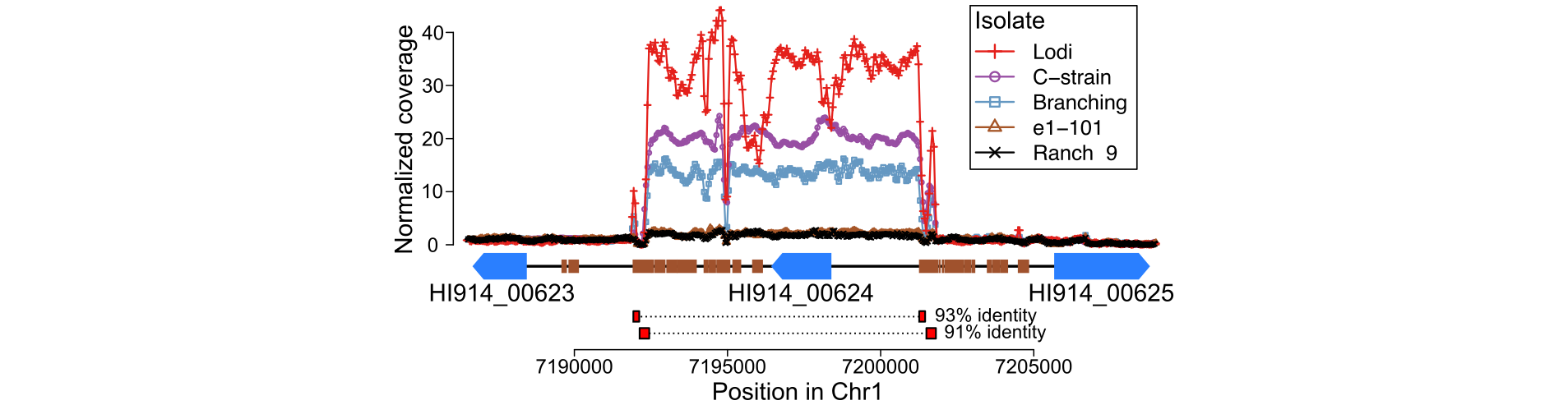
While investigating the genome of the grape powdery mildew Erysiphe necator for genes varying in copy number, I noticed that the gene with ID HI914_00624 was by far the one with highest copy number variation, between 1 and 30 predicted copies among five isolates analyzed. This alone sounded very interesting, and made the suspect that increase in copy number of this gene is likely a response to adaptation.
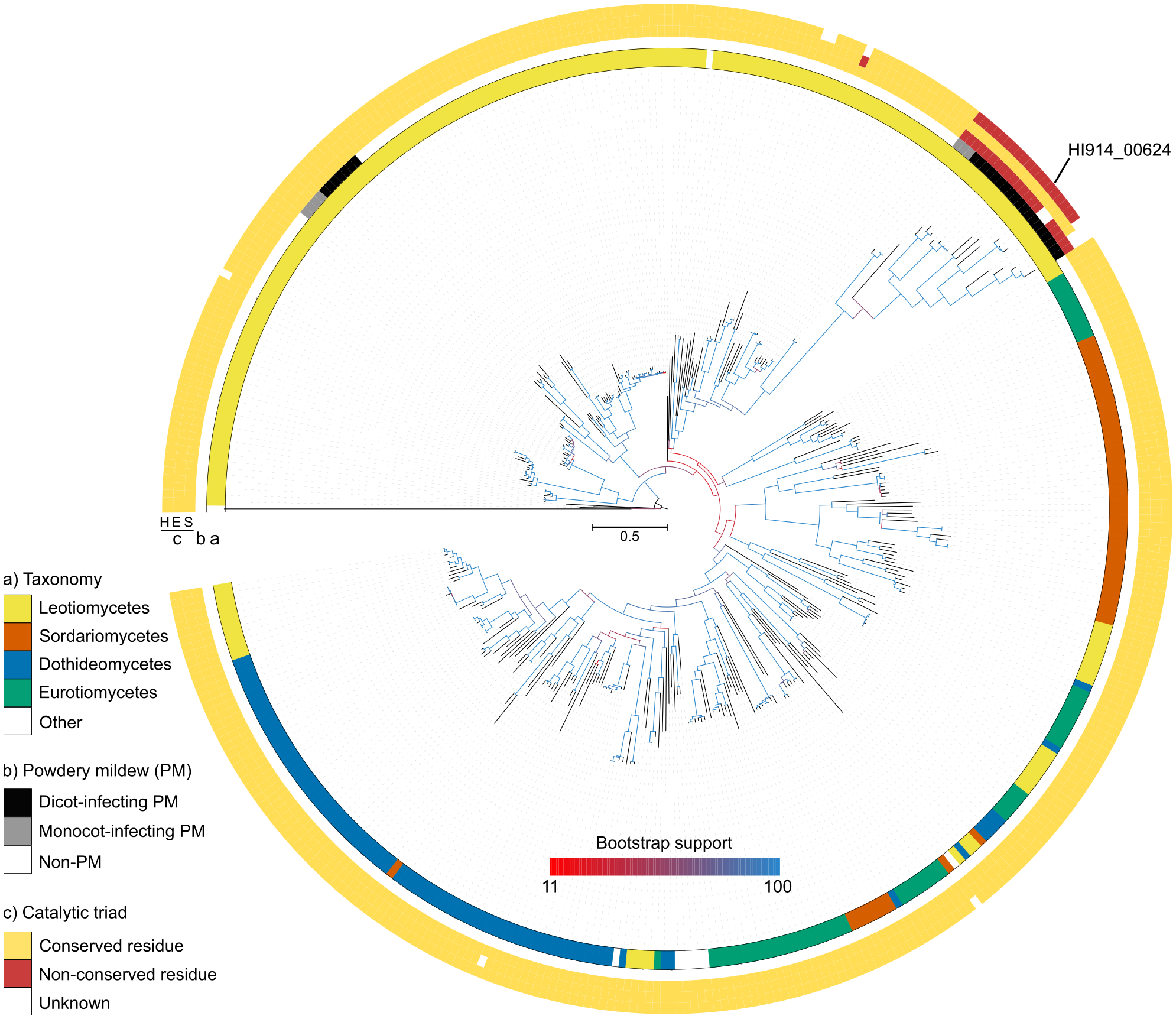
After further investigation, I predicted that the gene HI914_00624 encodes a secreted carboxylesterase. A phylogenetic tree revealed that homologs of this gene were present in other powdery mildew fungi. Together, they formed a separated branch far from carboxylesterases from other closely related species. Moreover, while comparing residues highly conserved among carboxylesterases, I noticed that two of the residues that comprise the catalytic triad were not conserved in HI914_00624 and its homologs in powdery mildew fungi. These observations made me speculate that HI914_00624 and its homologs in powdery mildew fungi form a new family of catalytically inactive carboxylesterases poorly conserved outside powdery mildews.
References
- Zaccaron, A. Z., Neill, T., Corcoran, J., Mahaffee, W. F., & Stergiopoulos, I. (2023). A chromosome-scale genome assembly of the grape powdery mildew pathogen Erysiphe necator reveals its genomic architecture and previously unknown features of its biology. Mbio, 14(4), e00645-23.
